If 2+2=47x that would’ve been a whole other story.
The class that will make you want to drop out of high school
shut your mouth
do you want this to be open for 20 years
Do you need help with MUN?
Also please help me with acids and bases
I hate tittytrations
so basically one kills the other resulting in water and some salt
yes I know that
but what about the calculations part with the pKa, pH, pOH, molarity, etc.
and log functions
thats the part i havent learnt and prob wont learn anytime soon lol
although i think ik about the molarity part
how to calculate molarity using titrations
all I remember is pKa = -logKa
tf is that
Titrations are used to find the molarity of an acid. Basically you have an acid in an erlenmeyer flask (of an unknown molarity) and a buret filled with sodium hydroxide (of a known molarity). Then you have to choose a specific pH indicator, which you put 2-3 drops of into the acid. Then, to pour the NaOH into the flask you have to turn the stopcock on the buret. Then you pour the NaOH into the acid until it changes color, but not too much or you’ll over-titrate it. Then you can use the known molarities, volume of acid, and volume of NaOH used to calculate out the molarity of the acid.
they’re very complicated to learn but once you get the hang of it, they’re pretty fun
If I can find an acid, NaOH, a beaker, an erlenmeyer flask, a buret, a buret stand, and a pH indicator lying around the house, I can demonstrate one for you
the formula you use to calculate the molarity of the acid is
M1V1 = M2V2
where M1 is the molarity of the acid, V1 is the volume of the acid (in mL), M2 is the molarity of the NaOH, and V2 is the volume of the NaOH used
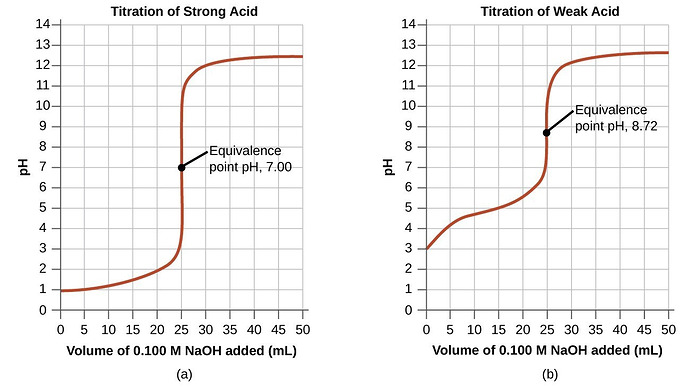
And you can also graph titrations, and they look like this
This is the worst part of the titration by far.
So there are some basic points you need to know
The first important point is the buffer zone. That’s basically the flat point at the bottom of the titration curve. Here, the pH of the solution remains constant.
The second point is the half equivalence point, which is exactly what it sounds like: half of the equivalence point. This is when there are equal concentrations of acid and base.
Then there’s the equivalence point, which is where the acid is neutralized and I have no idea how to find it
Then there’s the end point, where the curve flattens out at the top and the pH once again becomes constant.
To graph a titration all you need to do is stick a pH probe into the acid, download an app on your laptop to collect the data, connect the parts together, plug the pH probe into the laptop, set the laptop to collect data, and do the titration. However, do NOT let your pH probe EVER dry out. That will ruin the probe.
yeah i have 0 clue abt that
Yeah I think I can help with chemistry @Zach